The main preventative treatment for ‘recurrent UTIs’ is long-term low-dose antibiotics taken daily. Research has shown that they are effective at reducing further UTIs whilst they are taken. Unfortunately, once they are stopped, this benefit reduces.
The major concern with long-term low-dose daily antibiotics is the development of antibiotic resistance. Resistant UTIs can cause longer and more severe symptoms. However, the effect of low-dose antibiotic taken daily on resistance is not clear. There are also several non-antibiotic options, but how they compare to each other for effectiveness and side effects is also not clear.
The IMPART study will provide new information on both the positives and negatives of the various treatment options for ‘recurrent UTIs’. This important information will be developed, with patients, into a decision aid to support discussions between yourself and your healthcare professional about the best treatment for you. The stages, or work packages, of the IMPART study are presented below and the results of each stage will be summarised on the ‘Study news and updates’ tab throughout the course of the research
To achieve this, IMPART aims to answer the following questions:

What are the benefits and risks of long-term low dose antibiotics and non-antibiotic treatment options (e.g. methenamine or D-mannose) and how do they compare?

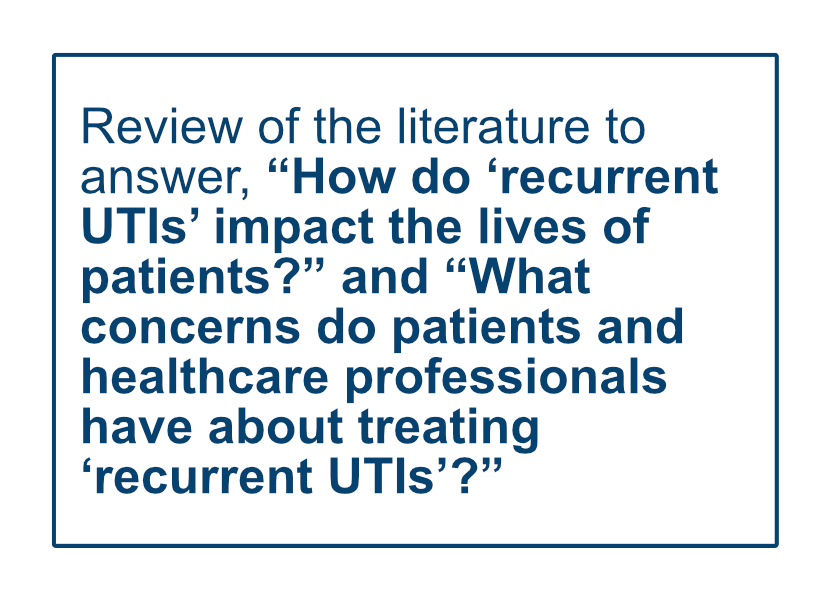
How do ‘recurrent UTIs’ impact the lives of patients? What concerns do patients and healthcare professionals have about treating ‘recurrent UTIs’?

How common are ‘recurrent UTIs’ and how often are long-term low dose antibiotics used?

What is the risk of long-term low dose antibiotics on developing resistance to antibiotics?

From a patient and healthcare professional perspective, what are the important aspects when discussing the management of recurrent UTIs?
Patient and public involvement
Funding
This study is funded by Welsh Government through Health and Care Research Wales.
See the Health and Care Research Wales page for project NIHR-FS-2021-LS (IMPART)
Research Summary
If you would like to find out more about the research methods used in the IMPART study, please go to the Research Summary Page
Research Plan














PROTOTYPE PATIENT DECISION AID DEVELOPMENT




















PROTOTYPE PATIENT DECISION AID DEVELOPMENT




© Copyright ImPART

